Dierexperimenteel onderzoek:
Jaarverslag 2022
De Rijksuniversiteit Groningen (RUG) en het Universitair Medisch Centrum Groningen (UMCG) voeren dierproeven uit voor onderzoek en onderwijs, omdat sommige belangrijke en relevante vragen niet te beantwoorden zijn zonder proefdieren.
We zijn daar open over en laten op deze website graag zien hoe we dierstudies doen en welke afwegingen we daarbij maken. Hiermee dragen we bij aan een maatschappelijke discussie over dierproeven waarin iedereen een weloverwogen mening kan vormen.
Nederlandse Transparantieovereenkomst Dierproeven
Het belang van goede en transparante communicatie over proefdieronderzoek wordt steeds breder onderschreven. De Rijksuniversiteit Groningen is een van de vijftien organisaties in Nederland de die Nederlandse Transparantieovereenkomst Dierproeven hebben ondertekend. Het doel van de Transparantieovereenkomst is het creëren van een opener en transparanter klimaat rondom dierproeven. De groep ondertekenaars bestaat uit universiteiten, universitaire medische centra, wetenschappelijke instituten, bedrijven en verenigingen die allen betrokken zijn bij dierproeven.
De ondergetekenden zijn: Amsterdam UMC, Biomedical Primate Research Centre, Charles River Laboratories Den Bosch B.V., Envigo RMS B.V., Erasmus Universitair Medisch Centrum, Koninklijke Nederlandse Akademie van Wetenschappen, Leiden Universiteit, Nederlands Kanker Instituut, Radboudumc, Radboud Universiteit, Rijksuniversiteit Groningen, Universiteit Maastricht, Vereniging Sportvisserij Nederland, Vrije Universiteit Amsterdam, Wageningen University & Research.
Transparantie
Het is wettelijk bepaald dat Nederlandse onderzoeksinstellingen informatie verstrekken over de dierproeven die ze doen, maar deze informatie is niet altijd voor iedereen toegankelijk en te begrijpen. Ondertekenaars hopen met deze overeenkomst een positieve bijdrage te leveren aan openheid rondom dierproeven.
Toezeggingen
De ondertekenaars zijn betrokken bij het uitvoeren, ondersteunen of financieren van dierproeven ten behoeve van de gezondheid van mens en dier, kwaliteit van leven en natuur en milieu. Door zich aan deze overeenkomst te verbinden doen de organisaties de volgende vier toezeggingen:
- We zijn duidelijk over wanneer, hoe en waarom we dieren in onderzoek gebruiken.
- We streven naar betere communicatie over dierproeven in Nederland met het publiek en de media.
- We bieden proactief mogelijkheden aan het brede publiek om kennis te maken met dierproeven en de regelgeving die daarop van toepassing is.
- Jaarlijks rapporteren wij onze voortgang en delen we ervaringen met elkaar.
Deze overeenkomst is opgesteld door Nederlandse onderzoekers in samenwerking met de European Animal Research Association (EARA) en Stichting Informatie Dierproeven (SID). De Nederlandse Transparantieovereenkomst Dierproeven is geïnspireerd op bestaande overeenkomsten die in België, Duitsland, Frankrijk, Portugal, Spanje en het Verenigd Koninkrijk zijn opgesteld.
Dierproeven in getallen
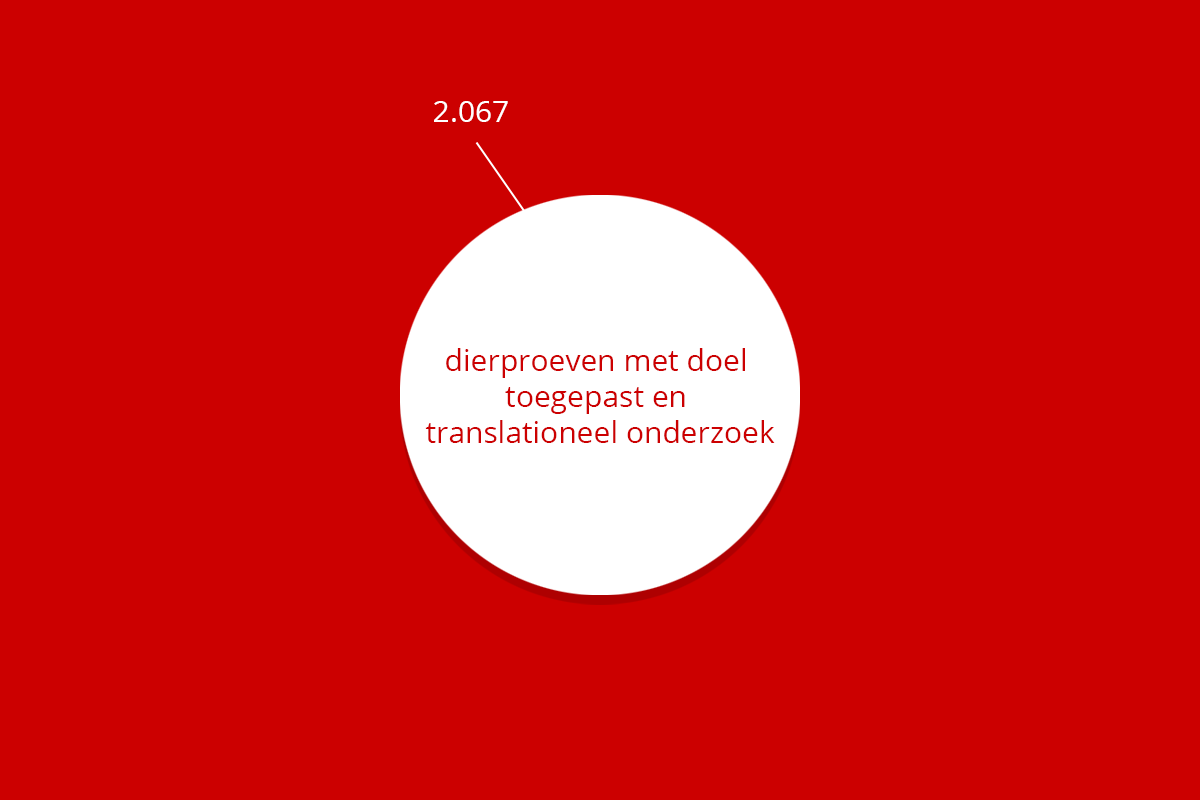
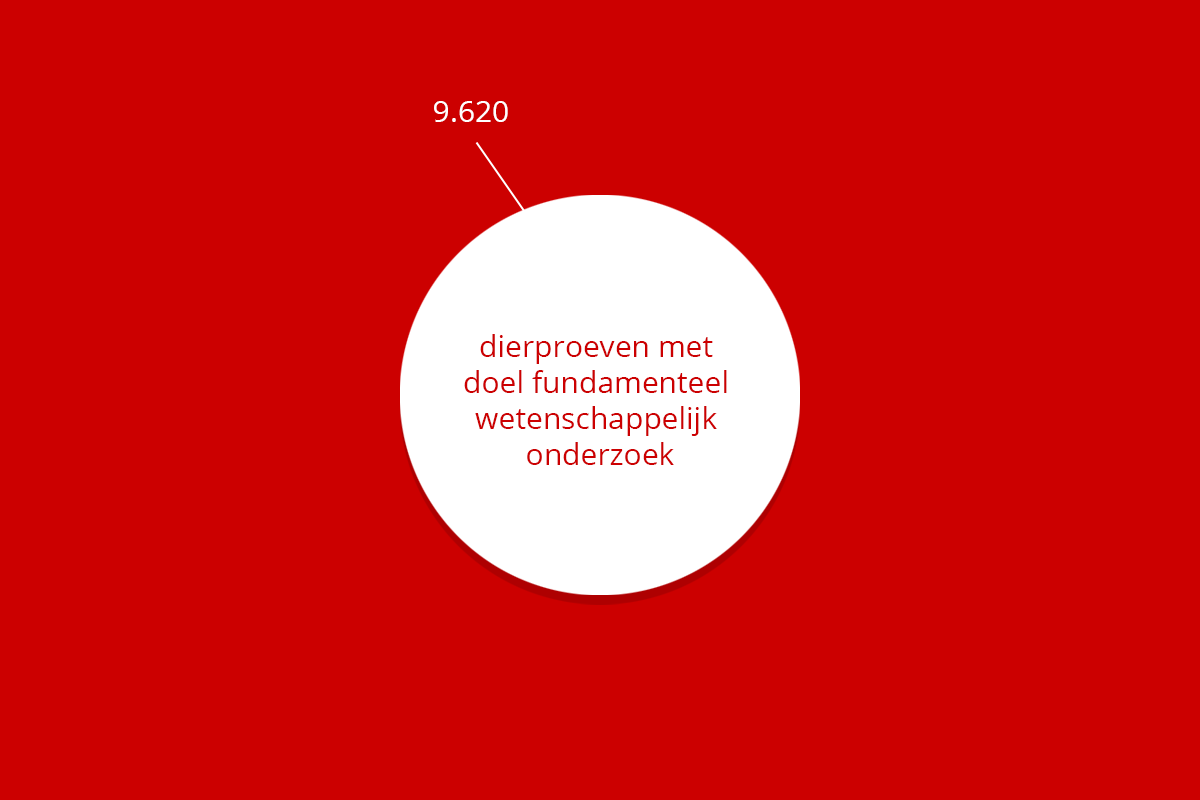
Binnen de RUG worden dierproeven uitgevoerd ten behoeve van fundamenteel en translationeel (toegepast) onderzoek alsmede voor onderwijs. Deze proeven vinden plaats in de faciliteiten bij respectievelijk het UMCG (de CDP) en FSE (de FDD) en ook vinden er proeven plaats in het vrije veld. In 2022 zijn in totaal 14.602 dierexperimenten verricht, waarbij voornamelijk gebruik werd gemaakt van muizen, ratten, vogels en vissen. In 2021 werden in totaal 16.473 dierexperimenten gedaan. In 2022 is dus een afname van bijna 2000 dierproeven (ongeveer 11%) te zien. Het aantal dierproeven fluctueert jaarlijks door beschikbare budgetten en onderzoekscapaciteit. Onderstaande figuur laat een overzicht zien van de aantallen uitgevoerde dierproeven uitgesplitst per diersoort en geeft de mogelijkheid de getallen van de laatste 5 jaar te vergelijken.
Er zijn bij de RUG jaarlijks grote schommelingen in het aantal proeven met muizen, maar de trend naar minder dierproeven met muizen lijkt nu door te zetten. Het aantal proeven met ratten daalt nog steeds. Dit is al sinds 2013 het geval. Dit jaar hebben we de zebravissen weer apart weergegeven van de andere vissen. In 2022 zijn geen proeven met zebravissen geregistreerd, omdat de meeste proeven met deze diersoort worden gedaan met vissenlarven, die niet als dierproef geregistreerd hoeven te worden als ze voor dag 5 (na het uitkomen van het ei) gebruikt zijn. De aantallen andere vissen zijn weer iets afgenomen; door de jaren is er steeds een grote variatie in de aantallen andere vissen gebruikt in dierproeven, maar voorlopig zullen de aantallen beperkt blijven want in 2022 is het onderzoek met Cichliden beëindigd en de dieren zijn door een andere instelling, buiten Nederland, overgenomen.
Het gebruik van andere vogels was in 2020 sterk afgenomen, wat zeer waarschijnlijk verband hield met beperkingen voor veldonderzoek t.g.v. de Corona-pandemie. Na een toename in 2021 is nu echter weer een sterke afname te zien. De aantallen ‘andere’ vogels gebruikt voor dierproeven fluctueren al jaren en waarschijnlijk blijft dit voorlopig zo. Dit jaar zijn pimpelmezen de meest gebruikte vogelsoort. Sinds in 2014 de herziene Wet op de Dierproeven is ingevoerd is het aantal dierproeven op de RUG met meer dan 25% gedaald en in de komende jaren zal duidelijk worden of deze lijn zich voort zal zetten. De ontwikkelingen van alternatieven voor dierproeven gaan ook door en hopelijk leidt dat de komende jaren ook tot meer afname van dierproeven.
Waarom dierproeven?
Gezond ouder worden (Healthy Ageing) en een duurzame samenleving (Sustainable Society) zijn kernthema’s in het beleid van het UMCG en de RUG. Veel onderzoeksprogramma’s richten zich dan ook op onderwerpen als gezonde veroudering, de ziekte van Alzheimer, diabetes en Parkinson, waarbij dierproeven soms noodzakelijk zijn. Maar ook om ecologische vraagstukken te ontrafelen, zoals het trekgedrag van vogels, zijn experimenten met dieren nodig.
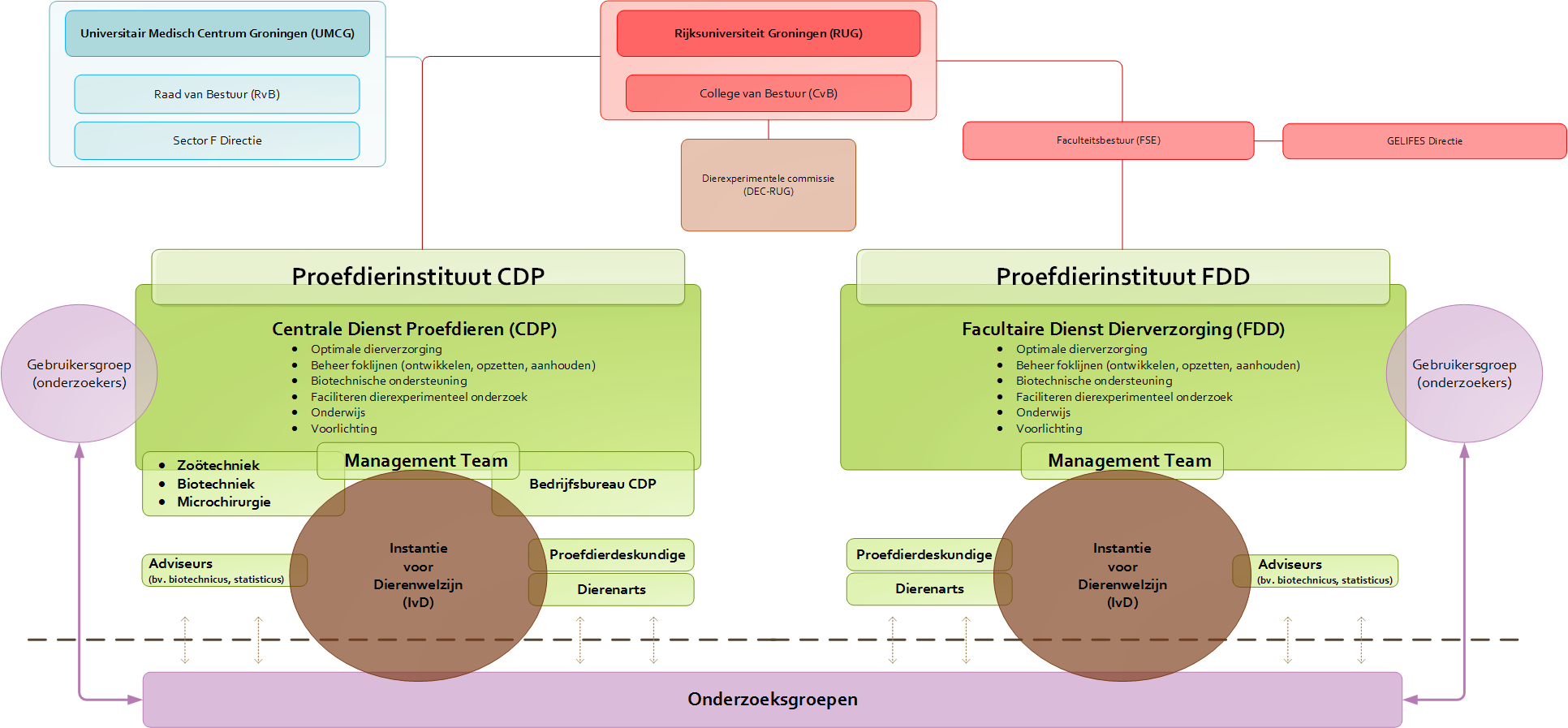
Dierproeven aan de RUG / UMCG
De RUG en het UMCG streven naar (fundamenteel) onderzoek en onderwijs dat tot de wereldtop behoort. Het dierexperimenteel onderzoek dat daar voor nodig is, willen we zo goed mogelijk uitvoeren: dat betekent een optimale verzorging en borging van het welzijn van de proefdieren én het optimaal faciliteren van de proefdieronderzoekers.
Ons dierexperimenteel onderzoek vindt plaats in het UMCG (65%) en bij de Faculteit Science and Engineering van de RUG (FSE, 35%). Binnen deze organisaties is een aantal onderzoeksinstituten waar het dierexperimenteel onderzoek voornamelijk plaatsvindt.
▶ Behavioural and Cognitive Neurosciences (CBN/BCN-BRAIN)
Fundamenteel en praktijkgericht onderzoek naar de werking van het (gezonde) brein, afwijkingen in het zenuwstelsel bij neurologische en geestelijke aandoeningen, en de mechanismen die ten grondslag liggen aan gedrag.
▶ Centre for Ecological and Evolutionary Studies (CEES)
Fundamenteel onderzoek naar onder andere diergedrag en ecofysiologie.
▶ Groningen Research Institute of Pharmacy (GRIP)
Fundamenteel en toepassingsgericht onderzoek naar geneesmiddelen.
▶ Groningen University Institute for Drug Exploration (GUIDE)
Lead-ontwikkeling en het ontwikkeling van geneesmiddelen.
▶ Health Research and Epidemiology (SHARE)
Opheldering van factoren die ervoor zorgen dat mensen op een gezonde manier oud worden (healthy aging) aan de hand van fundamenteel en praktijkgericht onderzoek.
▶ European Research Insitute for the biology of ageing (ERIBA)
Fundamenteel onderzoek naar de factoren die veroudering veroorzaken.
▶ Biomaterials (W.J.Kolff Institute)
Toepassings- en praktijkgericht onderzoek naar biomaterialen en implantaten.
▶ Fundamental, Clinical and Translational Cancer Research (Cancer Research Center Groningen)
Fundamenteel en praktijkgericht onderzoek naar oncologie en tumorontwikkeling.
De onderzoeksvoorbeelden in dit jaarverslag illustreren het onderzoek aan de RUG en het UMCG. Op de website van de RUG is een overzicht beschikbaar van de afdelingen die dierexperimenteel onderzoek uitvoeren.
De RUG en het UMCG zijn in dialoog met verschillende stakeholders op het gebied van dierproeven. Het doel hiervan is om zowel te zenden als te ontvangen. Zo wordt de dialoog gebruikt om de waarde van door de RUG en UMCG uitgevoerde dierproeven voor maatschappij en wetenschap goed uit te leggen. Anderzijds biedt het de mogelijkheid om signalen over dierproeven te ontvangen en binnen de organisatie een vervolg te geven.
Wet- en regelgeving
Proefdieronderzoek is aan strikte wet- en regelgeving gebonden. Sinds 1977 is het welzijn van proefdieren in Nederland beschermd via de Wet op de dierproeven, de Wod. In aanvulling op deze wet is sinds 1985 ook het Dierproevenbesluit van kracht. Uitgangspunt van de wet is het ‘Nee, tenzij’-principe: dierproeven zijn niet toegestaan, tenzij er geen alternatieven zijn. Wanneer een onderzoeker het onderzoek bijvoorbeeld ook met een computermodel of slachtmateriaal kan uitvoeren, is het verboden dieren voor het experiment te gebruiken. In 2014 is een herziene Wod in werking getreden.
Volgens de huidige Wod is een dierproef gedefinieerd als ‘elk al dan niet invasief gebruik van een dier voor experimentele of andere doeleinden, waarvan het resultaat bekend of onbekend is, of onderwijskundige doeleinden, die bij het dier evenveel of meer pijn, lijden, angst of blijvende schade kan veroorzaken als, dan wel het inbrengen van een naald volgens goed diergeneeskundig vakmanschap’. Proeven met dieren zonder inwendig skelet, zoals wormen, slakken en insecten, vallen niet onder dierproeven. De Wod is bedoeld om de proefdieren in Nederland te beschermen. Er staat bijvoorbeeld in dat alleen gekwalificeerde mensen proefdieren mogen gebruiken en dat alleen mogen doen binnen instellingen die daarvoor een vergunning hebben.

Codes of Practice
Onder de oude Wet op de Dierproeven werden voor het onderzoek met wilde dieren op het laboratorium en het onderzoek met wilde dieren in hun biotoop verschillende ‘dierproef-definities’ gehanteerd. Na invoering van de nieuwe Wod is dit onderscheid er niet meer en geldt voor al het dierexperimenteel onderzoek en dus ook voor dat met wilde dieren zowel binnen de instelling als in het biotoop dezelfde definitie. Al snel bleek dat onderzoek met wilde dieren in hun biotoop onderbelicht waren in de notitie ‘Wanneer is er sprake van een dierproef in de zin van de wet’ welke op 3 oktober 2016 is gepubliceerd op de website van de CCD. Daarop is een werkgroep opgericht met vertegenwoordigers uit het werkveld wat in 2017, in samenwerking met de CCD en de NVWA, heeft geresulteerd in de publicatie van de handreiking ‘Dierproeven met wilde dieren in hun biotoop’.
Onderzoekers van de RUG zijn betrokken geweest bij de totstandkoming van deze handreiking en de RUG gebruikt deze als leidraad bij aanvragen voor en uitvoering van onderzoek aan dieren in het vrije veld.
Van aanvraag tot uitvoer dierproef
CCD
Deze landelijke commissie beoordeelt de aanvraag definitief en verleent, of weigert, de projectvergunning voor het experiment. De CCD publiceert niet-technische samenvattingen van verleende vergunningen op haar website.
Daarnaast is een landelijk comité, het NCad opgericht. Het brengt verbeteringen tot stand gericht op het Vervangen, Verminderen en Verfijnen (3V’s) van dierproeven en de ethische toetsing daarvan in (toegepast) wetenschappelijk onderzoek en onderwijs om daarmee het proefdiergebruik te minimaliseren, zowel nationaal als internationaal.
DEC
De RUG heeft een onpartijdige dierexperimentencommissie die proefdiergebruik beoordeelt in opdracht van de CCD. Bij haar afweging gebruikt de DEC-RUG standpunten en richtlijnen die zijn opgesteld door de CCD. Daarbij hanteert de DEC-RUG ook de algemeen geldende standpunten uit de Code of Practice over diverse onderwerpen. In de DEC-RUG zitten deskundigen op het gebied van (bescherming van) proefdieren, dierproeven, alternatieven voor dierproeven en ethische toetsing.
De DEC toetst elk onderzoeksvoorstel aan de bestaande wet- en regelgeving. Ook weegt ze het belang van het dierexperiment af tegen het ongerief dat de proefdieren ondervinden.
De intrinsieke waarde van elk dier staat centraal bij de afweging of een dierproef ethisch acceptabel is of niet. Dat neemt niet weg dat ook andere overwegingen een rol spelen, zoals psychologische complexiteit van een dier (denk aan apen). Of de maatschappelijke status die aan een diersoort wordt toegekend, gebaseerd op factoren als sociale verbondenheid (hond en kat), historische waarde (landbouwhuisdieren) en maatschappelijke relatie (zeehond).
Voor proeven met apen zijn bij de RUG en het UMCG geen faciliteiten; over proeven met apen heeft de RUG een apart standpunt opgesteld.
IvD
Een belangrijke verandering in de herziene Wod is dat instellingen kennis over dierenwelzijn bundelen in een ‘Instantie voor Dierenwelzijn’. De IvD behandelt een onderzoeksproject, na goedkeuring door de DEC en CCD, op dierenwelzijnsaspecten en zorgt ervoor dat het project goed uitgevoerd kan worden. Verder adviseert zij de onderzoeker over toepassing van de 3V’s en houdt toezicht op de voorbereiding van het onderzoek en de vaardigheid en scholing van de uitvoerders.
In de IvD zit een aangewezen dierenarts, de locatiebeheerder van de dierfaciliteit, een wetenschapper en indien gewenst een externe deskundige, zoals bijvoorbeeld een stralingsdeskundige of een biologische veiligheidsfunctionaris.
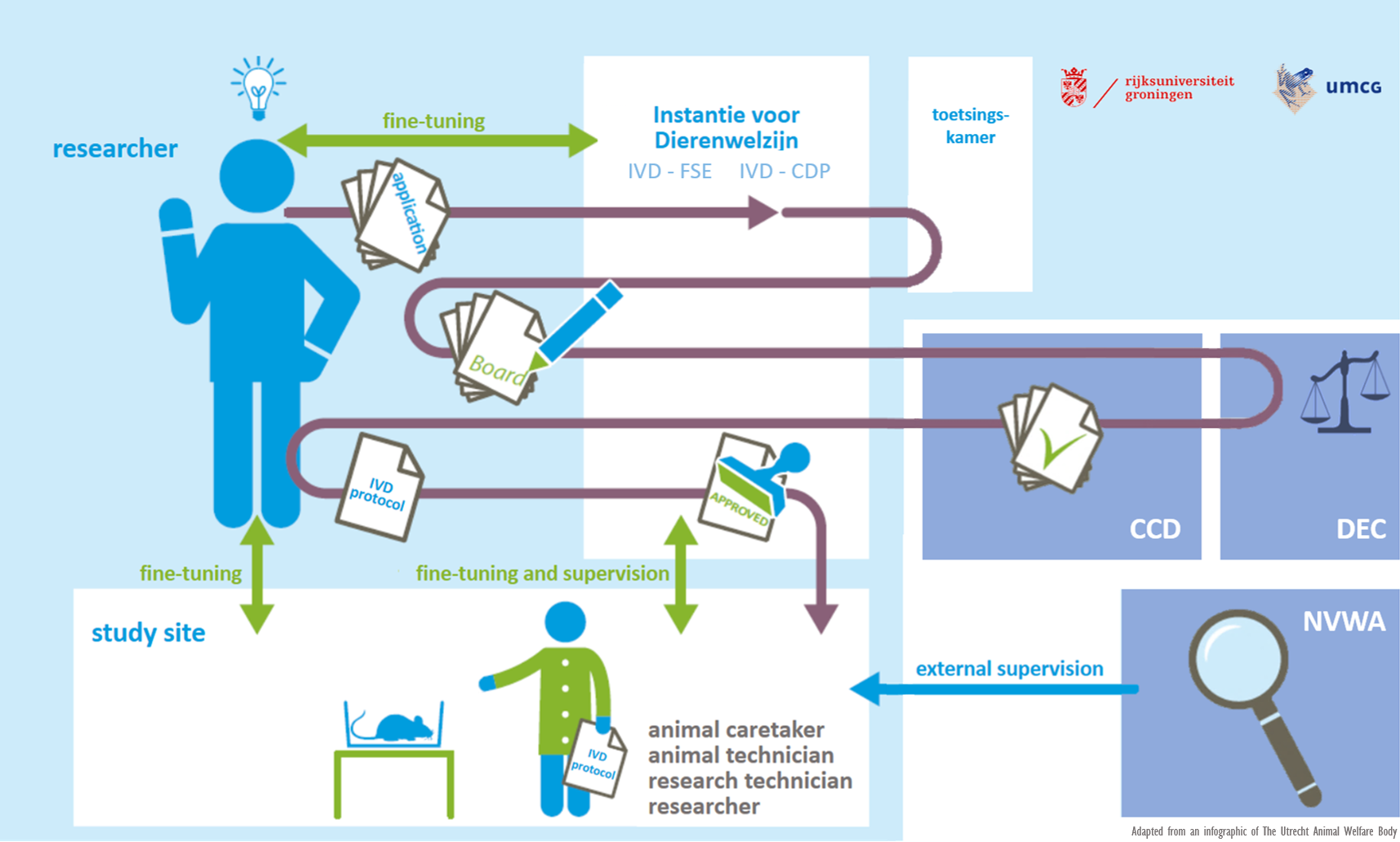
In de Wet op de dierproeven staan in artikel 14C. de taken van een Instantie voor Dierenwelzijn beschreven in vijf punten (14c.1a tot en met 1e). Lid 1c van het wetsartikel meldt dat de IvD “zorgt voor de vaststelling en toetsing van bedrijfsinterne procedures inzake controle, rapportage en vervolg met betrekking tot het welzijn van de in de inrichting gehuisveste dieren”. Vrij vertaald geeft het artikel aan dat de IvD organiseert dat het welzijn van de proefdieren geborgd is en wordt vastgelegd.
De IvD’s van de RUG checken bij elk IvD-protocol of de dierstudie uitgevoerd zal worden met dieren die in het wild worden gevangen. Als dit zo is, controleert de IvD of de benodigde Flora- en Fauna ontheffing aanwezig is. Dit bedrijfsinterne proces zal onveranderd gehanteerd blijven worden.
IvD platform
De beide IvD’s van de RUG zijn aangesloten bij het landelijk IvD-platform. IvD-Groningen is goed vertegenwoordigd binnen het IvD-Platform. We zijn niet alleen in verschillende werkgroepen actief (Werkgroepen Antibiotica en Fok Coördinatoren), er is ook 1 vast commissie lid binnen het Platform. Het IvD-platform dat onderdeel is van DALAS, was zeer actief in 2022. Tot op de dag van vandaag vertegenwoordigt het IvD-platform nog steeds meer dan 90% van alle IvD’s in Nederland.
Naast het maandelijkse reguliere overleg heeft het IvD-Platform contacten met de verschillende overheidsorganisaties zoals de CCD, de NCad, LNV en de NVWA. Het doel van het afgelopen jaar was om minimaal eenmaal per jaar met de verschillende overheidsorganisaties om de tafel te zitten om punten te bespreken die speelden op de werkvloer.
Naast gesprekspartner met de verschillende overheidsorganisaties, wil het IvD–Platform ook zorg dragen voor het delen van kennis met de IvD’s.
Binnen het IvD-platform zijn 4 werkgroepen actief: Antibiotica, Experimental Design & Statistical Analysis, Fok-coördinatoren en Intercollegiaal overleg. Output van deze werkgroepen zal gedeeld worden richting de werkvloer via de IvD’s.
Dit jaar heeft het IvD platform in samenwerking met de NCad 1x het “Harry Blom beraad” georganiseerd. Doel van dit beraad is om dilemma’s in de huidige onderzoekspraktijk vanuit verschillende gezichtspunten te belichten. Het onderwerp was dit keer ‘Het uitvoeren van natuurlijk gedrag van het dier heeft invloed op de dierproef”. Hierover gaven meerdere deskundigen hun onderzoeksbevindingen en visie.
Zelf heeft het IvD-Platform een middag georganiseerd voor IvD’s met als thema: ‘Horen, zien en praten met elkaar’. Hoe kunnen we samen onderzoeken en verbeteren!
Een ander belangrijk onderwerp binnen de IvD’s zijn de Codes of Practice (CoP’s). Daarom heeft het IvD-platform het initiatief genomen om samen met de NCad nieuwe CoP’s te ontwikkelen en oude CoP’s te herzien. Vanuit IvD-Groningen hebben twee personen deelgenomen aan de werkgroep Good Surgical Practice.
Einde van de proef
Adoptie
Volgens de herziene Wet op de dierproeven geeft de IvD advies over adoptieregelingen, met inbegrip van advies betreffende passende socialisatie van de voor adoptie vrij te geven dieren. De vergunninghouder is van mening dat het niet in het belang van de proefdieren en particulieren is om de proefdieren te laten adopteren. Een uitzondering hierop zijn dieren die bij de RUG onder semi-natuurlijke omstandigheden gehouden worden o.a. vogels en vissen. Deze dieren kunnen door particulieren overgenomen worden. De vergunninghouder staat niet toe dat dergelijke dieren verhandeld worden. In 2022 werden 4 Bankiva kippen, 21 Zebravinken, 43 Regenboogvissen, 2 Heevissen, 23 Kersenbuiken en 4 Maanvissen geadopteerd, 263 Stekelbaarzen werden vrijgelaten in de natuur.
Euthanasie
In de meeste gevallen is adoptie niet mogelijk, bijvoorbeeld omdat de hersenen en/of andere organen en lichaamsdelen nodig zijn voor verder onderzoek en analyse, en zullen dieren aan het einde van een dierproef gedood worden. Dit is een stap die geen van de dierverzorgers en onderzoekers graag uitvoert.
De euthanasieprocedure die voornamelijk gebruikt wordt is zo ontwikkeld dat de dieren er zo min mogelijk van merken. De dieren komen in een bak met een mengsel van zuurstof (O2) en koolstofdioxide (CO2), waarbij het CO2-gehalte langzaam oploopt. Hierdoor raken de dieren eerst buiten bewustzijn, waarna ze langzaam inslapen. Soms is het, in het kader van de proef, wenselijk om te kiezen voor een andere euthanasieprocedure. Ook in dat geval zal er altijd op worden toegezien dat de gekozen methode zo weinig mogelijk ongerief veroorzaakt voor het dier.
In enkele gevallen krijgen dieren tijdens een experiment complicaties waardoor de dieren meer dan verwacht (dreigen te) lijden. In zulke gevallen passen onderzoekers het zogenaamde ‘humaan eindpunt’ toe. Ze halen het dier uit het experiment op het moment dat het lijden onacceptabel dreigt te worden. Het dier wordt vervolgens geëuthanaseerd om ernstig leed te voorkomen.
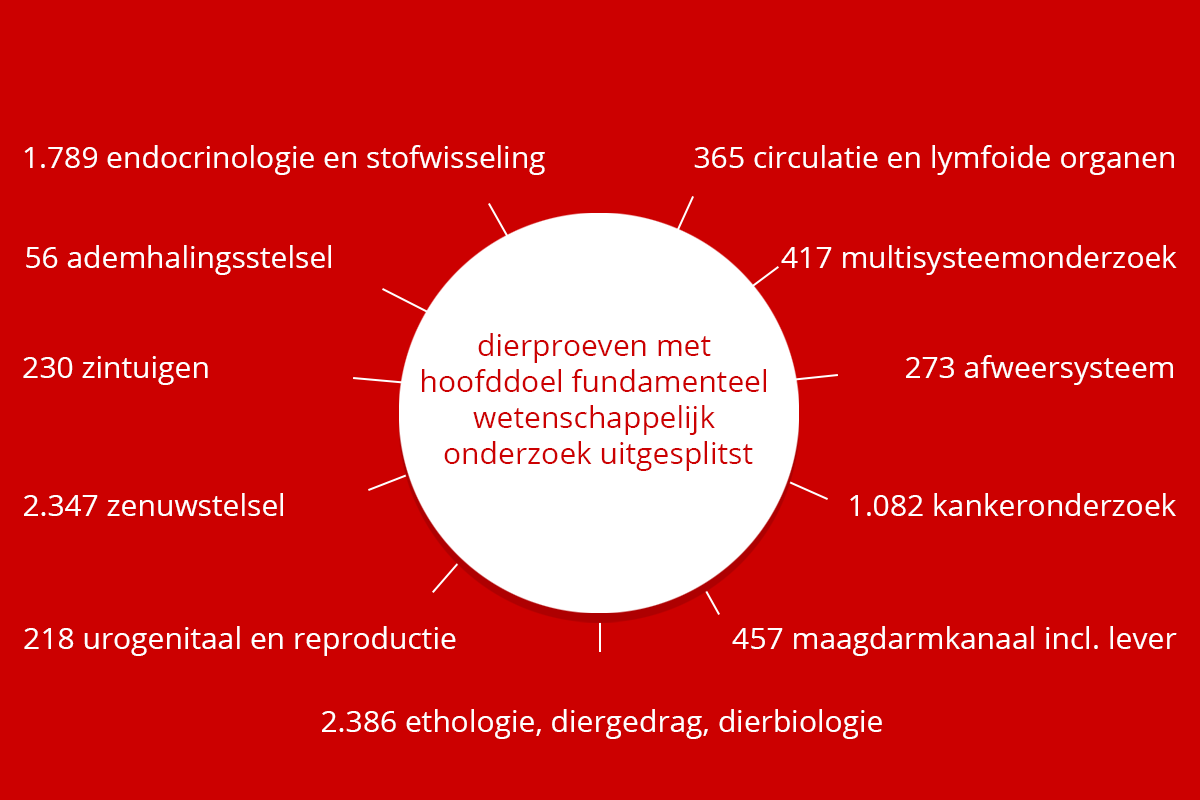
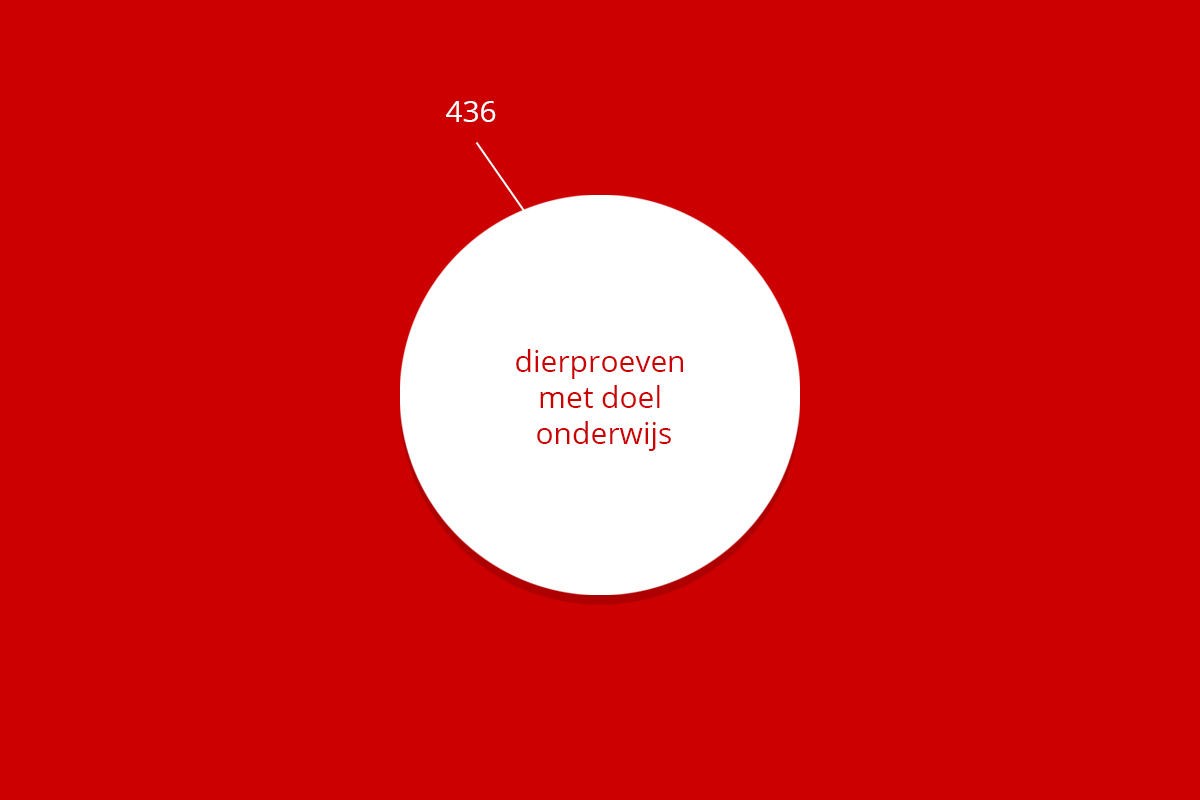
Dierproeven doelen
Veruit de meeste dierproeven hebben onderzoekers uitgevoerd om een wetenschappelijke vraag te beantwoorden. De figuur hieronder geeft aan waarover deze vragen gingen. Naast het beantwoorden van wetenschappelijke vragen, zijn ook dierproeven gedaan voor onderwijs en training, bijvoorbeeld om studenten en biotechnici op te leiden.
Tabel Zodoende
| RuG 10500 | Fundamenteel wetenschappelijk onderzoek | Toegepast en omzettingsgericht onderzoek tbv de Mens | Bescherming van diersoorten | Onderwijs | Fok met ongerief, niet gebruikt in dierproef | Totaal |
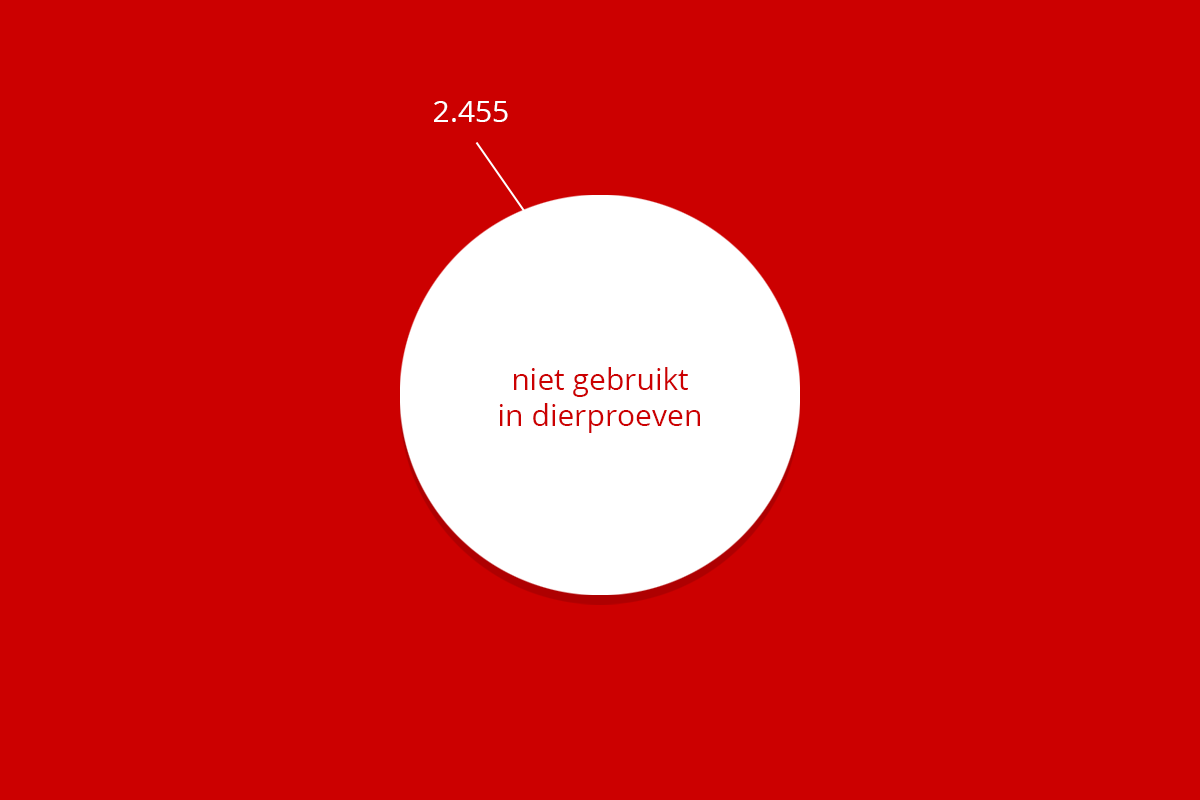
| Muizen | 6.055 | 1.796 | 202 | 2.455 | 10.508 | |
| Ratten | 1.088 | 61 | 232 | 1.381 | ||
| Cavia’s | 210 | 2 | 212 | |||
| Andere knaagdieren | 126 | 126 | ||||
| Andere zoogdieren |
5 | 5 | ||||
| Andere vogels | 1.419 | 24 | 1.443 | |||
| Xenopus |
111 | 111 | ||||
| Zebravissen | 0 | |||||
| Andere vissen | 816 | 816 | ||||
| Totaal | 9.620 | 2.062 | 24 | 436 | 2.455 | 14.602 |
NB lege rijen en kolommen worden niet getoond.
De tabel bevat geen dieren die zonder voorafgaande handeling zijn gedood.
Ongerief
Proefdieren ervaren altijd een bepaalde mate van ongerief. De Nederlandse regelgeving deelt ongerief met ingang van de herziene Wod in vier categorieën in. Ongerief hoeft niet per se pijn te zijn: ook stress en angst vallen hieronder. Hieronder is weergegeven welke mate van ongerief de dierproeven in 2022 met zich meebrachten.
%
Terminale dierproeven
%
Licht ongerief
%
Matig ongerief
%
Ernstig ongerief
%
Ernstig overstijgend ongerief
Fokefficiëntie
De RUG en het UMCG fokken zelf dieren, vooral (transgene) muizen en ratten. Niet alle gefokte dieren komen in een experiment terecht. Deze dieren noemen we ‘surplusdieren’ of ‘fokoverschot’.
Een fokoverschot is helaas onvermijdelijk. Dieren in een proef moeten vaak zoveel mogelijk identiek zijn om betrouwbare onderzoeksresultaten te krijgen. Ze moeten bijvoorbeeld even oud en van hetzelfde geslacht zijn of onder identieke omstandigheden zijn geboren. Ook bezitten niet alle dieren de gewenste genetische eigenschappen. Zo zijn voor een experiment met 60 identieke transgene muizen al snel 170 dieren gefokt: lees meer hierover op de website van de Stichting Informatie Dierproeven. Verder is een aanzienlijk deel van de fok nodig voor het in stand houden van unieke of waardevolle foklijnen.
Het terugdringen van het aantal dieren dat wel gefokt is maar niet in een experiment is gebruikt, heeft voor de RUG en voor de landelijke overheid, een hoge prioriteit. Er is in 2022, net als in 2020 en 2021, ongeveer een vijfde van de gefokte dieren geleverd voor experimenten. Bij niet-transgene lijnen is dit percentage hoger, omdat daar alle nakomelingen ook bruikbaar kunnen zijn voor het experiment. Bij transgene lijnen heeft een deel van de nakomelingen een genotype dat niet bruikbaar is in experimenten. Het totale aantal gefokte dieren (muizen en ratten) is in 2022 ruim 4000 lager dan in 2021 en het absolute aantal gefokte dieren dat is afgevoerd zonder in een experiment gebruikt te zijn gedood in voorraad, is ongeveer 5.000 dieren lager dan in 2021 en inmiddels al 15.000 lager dan in 2020. Dit gaat de goede kant op, maar de RUG is zich er terdege van bewust dat het aantal niet gebruikte dieren nog steeds te hoog is en dat meer inspanningen geleverd blijven worden om het aantal dieren gedood in voorraad te verminderen.
De RUG en het UMCG lopen voorop in het verhogen van de fokefficiëntie door een juiste communicatie tussen onderzoekers en dierverzorgers (vraag en aanbod) en het terugdringen van het fokoverschot door in te zetten op cryopreservatie van foklijnen die niet langer actief gebruikt worden.

Cryopreservatie
Cryopreservatie is een techniek waarbij eicellen of sperma van een foklijn die langere tijd niet nodig is, wordt ingevroren in plaats van de lijn levend in stand te houden. Als de foklijn weer nodig is, wordt een bevruchte eicel ingebracht bij een schijnzwanger vrouwtje. In de tussentijd zijn dus geen dieren nodig voor het behoud van de lijn.
Sinds 2020 biedt de RUG aan om inactieve foklijnen (lijnen die niet gebruikt worden voor dierproeven) te cryopreserveren. Achtentwintig muizenlijnen zijn gestopt in 2022. Zes nieuwe lijnen zijn gestart in 2022. Deze aantallen liggen veel lager dan vorig jaar, waarschijnlijk omdat in de afgelopen twee jaar, toen de optie van cryopreservatie beschikbaar kwam, al meer lijnen gestopt zijn die de jaren daarvoor langer zijn aangehouden.
Voor vissenlijnen is het nog niet mogelijk genetisch materiaal in te vriezen en later weer te gebruiken om vissen te kweken. Dit wordt wel uitgeprobeerd bij diverse instituten en de RUG zal deze trend goed in de gaten houden om ook cryopreservatie van vissenlijnen in de toekomst mogelijk te maken. Al met al zijn we nog niet tevreden met deze cijfers en hopen het percentage ongebruikte dieren de komende jaren te verlagen.
Vervanging, Vermindering en Verfijning
Bij onderzoek en onderwijs met proefdieren aan de RUG en het UMCG staan de 3V’s centraal: vervanging en vermindering van proefdieren en verfijning van de dierproeven. Concreet betekent dit dat we zo min mogelijk proefdieren gebruiken en waar mogelijk proefdiervrij werken. Het ongerief voor de proefdieren beperken we zo veel als mogelijk. De Instantie voor Dierenwelzijn (IvD) helpt de onderzoekers om dit in de praktijk te brengen.
Vervanging
Een onderzoeker mag een dierproef alleen uitvoeren als het niet anders kan. Waar mogelijk voeren we onderzoek en onderwijs uit met dierproefalternatieven zoals ongewervelden, cellen, weefsels, computersimulaties, videotraining of slachthuismateriaal.
Vermindering
Bij een dierexperiment zetten we in op het verminderen van het aantal benodigde dieren: een opzet met zo min mogelijk proefdieren, die nog wel betrouwbare resultaten oplevert. Bijvoorbeeld door te kiezen voor standaardstammen waardoor de onderzoeksresultaten beter vergelijkbaar zijn of door onderzoekers eerst een pilotonderzoek te laten uitvoeren.
Soms kunnen proefdieren na het oorspronkelijke experiment opnieuw worden gebruikt, voor een (vervolg)experiment of voor onderwijs. In 2022 ging 3,5% van de dieren een tweede maal een proef in.
Verfijning
De onderzoekers, dierverzorgers, biotechnici en proefdierdeskundigen zijn dagelijks met verfijning bezig. Optimale huisvesting en goede toepassing van experimentele technieken en anesthesie, beperken het ongerief voor de proefdieren. Sociale dieren als ratten zijn bijvoorbeeld gehuisvest in groepen, waardoor ze minder stress ervaren.
Door dierproeven te verfijnen neemt het welzijn van de dieren toe. Goed voor de dieren en voor de kwaliteit van het onderzoek.
Toepassing 3V’s in het onderwijs met proefdieren
Training
Bij de Centrale Dienst Proefdieren worden bij het gebruik van proefdieren in het onderwijs zoveel mogelijk de 3V’s toegepast. Wanneer onervaren personen voor het eerst voor een training komen wordt zo veel mogelijk gebruik gemaakt van kunstmaterialen. Het leren hechten start met hechten op een zeemleren lap.
Wanneer iemand microchirurgische technieken wil leren, traint men eerst op een stukje latex handschoen onder de microscoop, vervolgens op kunstvaten en bij voldoende ontwikkeling van de oog-handcoördinatie, gaat men verder op de levende rat. Er wordt zoveel mogelijk gebruik gemaakt van kunstmaterialen, maar uiteindelijk zal de te leren techniek toch uitgevoerd moeten worden in een levend organisme, want een levend dier is een te complex geheel van factoren om deze na te bootsen met kunstmaterialen. Om het aantal gebruikte proefdieren te verminderen zijn er instructiefilms gemaakt van alle relevante biotechnische ingrepen die tijdens trainingen aan bod komen, zodat er geen dieren gebruikt hoeven te worden om een techniek voor te doen. Proefdieren die gebruikt worden in het kader van onderwijs krijgen bij invasieve operatieve ingrepen altijd pijnstilling en worden na afloop van de ingreep getermineerd onder narcose om onnodig ongerief te voorkomen. Door bij dierproeven betrokken personen goed op te leiden streeft de CDP ernaar om de kwaliteit van dierproeven en het welzijn van proefdieren te verbeteren.
Anatomiepracticum
In de bachelor fase van hun studie nemen alle biologie‐studenten deel aan een practicum anatomie en fysiologie waarbij een rat ontleed wordt. Tot 2015 werden ratten altijd kort voor aanvang van het practicum geëuthanaseerd en onbehandeld voor het practicum aangeboden, omdat dit het mooiste preparaat opleverde. In bevroren en vervolgens weer ontdooide preparaten bleken bepaalde essentiële structuren niet goed zichtbaar. Binnen de RUG zijn gedurende het jaar genoeg surplus dieren beschikbaar vanuit fok en (non‐invasieve) experimenten om te voorzien in de behoefte voor het practicum. Het is echter vanuit het oogpunt van welzijn onwenselijk om deze dieren voor langere tijd aan te houden tot de start van het practicum. Daarnaast betekent het lang aanhouden van dieren een belasting van de bedrijfsvoering. Om die reden werden tot 2015 de gebruikte ratten betrokken van een commercieel fokbedrijf. Ondanks dat de gebruikte dieren vrijwel uitsluitend surplusdieren waren uit de verschillende foklijnen bleef deze situatie sub‐optimaal, niet in de laatste plaats door de stress voor de dieren door bijvoorbeeld het transport.
In 2016 is om bovenstaande redenen begonnen met een zeer succesvolle pilot om ratten die gedurende het jaar overbleven uit de eigen fok en experimenten te balsemen. De gebalsemde ratten zijn zeer goed bruikbaar gebleken voor het practicum doordat alle belangrijke structuren goed behouden bleven in tegenstelling tot de ingevroren preparaten.
Voor het balsemen is gebruik gemaakt van de “Fix for Life” ‐ methode, ontwikkeld door het Leids Universitair Medisch Centrum. De methode maakt gebruik van een balsemingsvloeistof zonder, of met hele lage concentratie van giftige en irriterende stoffen die doorgaans nodig zijn (formaldehyde en fenol) voor weefselconservering. Hierdoor is deze methode uitermate geschikt voor onderwijsdoeleinden,voordeel hierbij is ook dat de balsemingsvloeistof aangenamer ruikt.
Het balsemen van de ratten is een win‐win oplossing gebleken. In de eerste plaats hoeven voortaan geen ratten meer aangeschaft en getransporteerd te worden en is er een nuttig doel gevonden voor de eigen surplusdieren. Tegelijkertijd zijn de preparaten uitermate geschikt voor het practicum en blijkt het gebruik ervan minder belastend voor de studenten.
Organisatie en faciliteiten
Om een optimale verzorging van de dieren te garanderen en effectief onderzoek te kunnen doen, zijn twee moderne proefdierfaciliteiten ingericht: de Centrale Dienst Proefdieren in het UMCG (CDP) en de Facultaire Dienst Dierverzorging in de Linnaeusborg (FDD).
Het proefdieronderzoek aan de RUG en het UMCG vindt plaats in de vrije natuur of in één van de laboratoria met speciale proefdierfaciliteiten. We besteden de grootst mogelijke zorg aan een optimale huisvesting van de proefdieren: dit is immers de ruimte waar de dieren vrijwel hun hele leven verblijven. Huisvesting is dan ook meer dan enkel voldoen aan de wettelijke vereisten. De CDP en de FDD zijn geheel vernieuwd in respectievelijk 2009 en 2011 en behoren tot de modernste van Europa. Temperatuur, licht en luchtvochtigheid in de verblijven zijn nauwkeurig te regelen.
Inspecties door NVWA
In 2022 heeft de NVWA geen inspecties uitgevoerd bij de dierfaciliteiten.

Over de Rijksuniversiteit Groningen
De Rijksuniversiteit Groningen is een mondiaal georiënteerde research universiteit, geworteld in Groningen, City of Talent. De Universiteit bevindt zich op invloedrijke ranglijsten in de top 100 en is geliefd bij studenten (30.000) en medewerkers (5250 fte) uit binnen- en buitenland. Zij worden uitgedaagd het beste uit zichzelf te halen; talent krijgt de ruimte, kwaliteit staat centraal. De universiteit werkt actief samen met maatschappelijke partners en profileert zich op de thema’s Healthy Ageing, Energy en Sustainable Society.