Animal studies:
Annual report 2022
The University of Groningen (UG) and the University Medical Center Groningen (UMCG) conduct animal experiments within the scope of research and teaching, because some important and relevant questions cannot be answered without the use of laboratory animals.
We are open about these activities and have created this website to show how we conduct animal studies and what we take into consideration in such testing. This is our contribution to the social debate about animal experiments in which anyone can form a considered opinion.
Dutch Transparency Agreement on Animal Testing
The importance of good and transparent communication about animal research is advocated by an increasing number of people. The University of Groningen is one of 15 organizations in the Netherlands that have signed the Netherlands Transparency Agreement on animal research. The aim of this Transparency Agreement is to create a more open and transparent climate around animal research. The signatories comprise universities, university medical centres, scientific institutes, companies, and associations that are all involved in animal research.
The signatories are Amsterdam UMC, Biomedical Primate Research Centre, Charles River Laboratories Den Bosch B.V., Envigo RMS B.V., Erasmus University Medical Center, the Royal Netherlands Academy of Arts and Sciences, Leiden University, the Netherlands Cancer Institute, Radboudumc, Radboud University, the University of Groningen, Maastricht University, Sportvisserij Nederland (the Dutch Angling Association), VU Amsterdam, and Wageningen University & Research.
Transparency
It is determined by law that Dutch research institutes provide information about the animal research that they carry out; however, this information is not always accessible and understandable for everyone. The signatories hope that this agreement will make a positive contribution to the openness around animal research.
Commitments
The signatories are involved in carrying out, supporting, or funding animal experiments for the benefit of human and animal health, quality of life, and nature and the environment. By signing this agreement, the organizations have made the following four commitments:
- We are clear about when, how, and why we use animals in research.
- We aim to improve the communication about animal research in the Netherlands with the public and with the media.
- We will be proactive in providing opportunities for the public to inform themselves about animal research and the regulations that govern it.
- We will report on our progress annually and share our experiences.
This agreement has been drawn up by Dutch researchers in collaboration with the European Animal Research Association (EARA) and Stichting Informatie Dierproeven (SID, the Animal Research Information Foundation). The Netherlands Transparency Agreement on animal research is based on existing agreements that have been drawn up in Belgium, Germany, France, Portugal, Spain, and the United Kingdom.
Animal testing statistics
Within the UG, animal research is carried out for the benefit of fundamental and translational research, as well as for educational purposes. The experiments are carried out at the UMCG facility (CDP) or at the FSE facility (FDD, Faculty Department of Animal Care), while some experiments are carried out in the open field. In 2022, a total of 14,602 animal experiments were carried out, mainly using mice, rats, birds and fish. In 2021, a total of 16,473 animal experiments were conducted. In 2022, a decrease of almost 2000 animal experiments (about 11%) can be seen. The number of animal experiments shows an annual fluctuation due to available budgets and research capacity. The table below shows an overview of the number of animal experiments that were carried out, categorized according to animal species, and has the option to compare the numbers for the past five years.
At the UG, the number of experiments on mice shows large annual fluctuations, but the trend towards fewer animal experiments with mice now seems to be continuing. The number of experiments on rats continues to decrease, as it has since 2013. We show the zebrafish statistics separately from the other fish. In 2022, no experiments with zebrafish were registered, because most experiments with this species are done with fish larvae, which do not have to be registered as animal experiments if they have been used before day 5 (after the hatching of the egg). The numbers of other fish have decreased slightly; over the years there has always been a large variation in the numbers of other fish used in animal experiments, but for the time being the numbers will remain limited because in 2022 the research with Cichlids ended and the animals were transferred to another institution, outside the Netherlands.
The use of other birds decreased sharply in 2020, which was most likely related to field research restrictions due to the Corona pandemic. However, following an increase in 2021 the numbers now show a sharp decrease again. The numbers of ‘other’ birds used for animal testing have fluctuated for years and will probably remain so for the time being. This year, blue tits are the most commonly used bird species.
Since the revised Animal Experiments Act was introduced in 2014, the number of animal experiments at the University of Groningen has decreased by more than 25% and in the coming years it will become clear whether this trend will continue. The development of alternatives to animal testing will also continue and hopefully this will also lead to a further decrease in animal testing in the coming years.
Why animal experiments?
Staying healthy while getting older (Healthy Ageing), adapting to changing circumstances (Adaptive Life) and creating a robust society (Sustainable Society) are policy spearheads of the UMCG and the UG. Many of our research programmes therefore focus on issues such as healthy ageing, Alzheimer’s disease, diabetes and Parkinson’s disease, which sometimes require animal testing. Animal experiments are also required to study ecological phenomena such as bird migration.
Animal experiments at the UG/UMCG
The University and the UMCG want their fundamental and applied research programmes to be among the best in the world. We wish to conduct the animal studies required to achieve this goal in the best possible manner, which means providing optimal care to lab animals and safeguarding of their welfare as well as optimal facilitation of the animal experimenters.
Our animal tests are conducted at the UMCG (65%) and the Faculty of Science and Engineering (FSE, 35%), where animal testing is concentrated in several research institutes.
▶ Behavioural & Physiological Ecology Group
Research into the behaviour of animals in their natural surroundings
▶ Conservation Ecology Group
Research into the impact of habitat changes on organisms
▶ Theoretical Research in Evolutionary Life Sciences (TRES)
Focus on theorical developments in evolutionary ecology, behavioural sciences and evolutionary systems biology
▶ Evolutionary Genetics, Development & Behaviour (EGDB)
Research into the proximate cause of phenotypic diversity and its ecological and evolutionary consequences
▶ Genomics Research in Ecology & Evolution in Nature (GREEN)
Research into ecological, evolutionary and conservationist issues in relation to biodiversity, ecosystems, living environment interactions, speciation, adaptation and plasticity
▶ Neurobiology
Research into the role of the brain in the capacity of animals and humans to adapt to challenges and opportunities in the environment
▶ Groningen Research Institute of Pharmacy (GRIP)
Fundamental and applied pharmaceutical research
▶ Groningen University Institute for Drug Exploration (GUIDE)
Development of new medication
▶ Health Research and Epidemiology (SHARE)
Fundamental and applied research into factors that help people to stay healthy while getting old (Healthy Ageing)
▶ European Research Insitute for the Biology of Ageing (ERIBA)
Fundamental research into factors causing ageing
▶ Biomaterials (W.J.Kolff Institute)
Applied research into biomaterials and implants
▶ Fundamental, Clinical and Translational Cancer Research (Cancer Research Center Groningen)
Fundamental and applied research into oncology and tumour development
The research examples in this annual report illustrate research at the UG and the UMCG. An overview of the departments that conduct animal experimental research is available on the UG website.
The University of Groningen and the UMCG are in dialogue with various stakeholders in the field of animal testing. Its purpose is to both share and exchange information. For example, the dialogue is used to properly explain the value of animal experiments performed by the University of Groningen and UMCG to society and science. On the other hand, it offers the possibility to receive signals about animal testing and to follow up within the organization.
Legislation and regulations
Animal studies are governed by strict legislation and regulations. Since 1977, the welfare of lab animals used in the Netherlands has been protected by the Wet op de dierproeven (Wod). To supplement this act, the Dierproevenbesluit (Animal Experiments Decree) was adopted in 1985. The principle underlying the act is the ‘No, unless’ principle: animal experiments are only allowed if there are no alternatives. If researchers can conduct a study by using a computer model or slaughterhouse material, for example, they will not be allowed to use animals for their experiments.
With the Wod, the Netherlands had good legislation governing the use of lab animals. There were major differences with other countries, however, including European member states. To achieve identical legislative standards – at least within the European Union – guidelines were drafted, which in the Netherlands led to a revision of the Wod in 2015. The current Wod defines an animal experiment as ‘any use, invasive or non-invasive, of an animal for experimental or other scientific purposes, with known or unknown outcome, or teaching purposes, which may cause the animal a level of pain, suffering, anxiety or lasting harm equivalent to, or higher than, that caused by the introduction of a needle according to good veterinary practice’. Experiments conducted on animals without an endoskeleton, such as worms, snails and insects, are not covered by the Wod. The intention of the Wod is to protect lab animals in the Netherlands. One of its clauses stipulates, for example, that only qualified personnel are allowed to use lab animals and only within institutions that have a permit for such use.
In the old Experiments on Animals Act, two definitions were used for research involving wild animals: one covering their use in the laboratory and one covering animals living in nature. This distinction is no longer made in the newWod, which means that the same definition covers all animal testing, including research involving wild animals, whether in the laboratory or in their biotopes. It soon appeared that research involving wild animals in their biotopes was not covered in sufficient depth in the memorandum with the title ‘Wanneer is er sprake van een dierproef in de zin van de wet?’ (‘When is an experiment an animal experiment under the Act?’, in Dutch only) which was published on theCCDwebsite on 3 October 2016. A project group was therefore established with representatives from the relevant fields. In collaboration with theCCDand theNVWA, in 2017 the group published guidelines with the title ‘Dierproeven met wilde dieren in hun biotoop’ (‘Animal experiments with wild animals in their biotopes’, in Dutch only). UG researchers were involved in the formulation of these guidelines, which are used by UG researchers applying for and implementing animal experiments in nature.

Codes of Practice
Although legislation provides frameworks, it does not concern itself with details. For this reason, its specific interpretation may be unclear. Experts have therefore drafted several Codes of Practice covering various research fields: ‘Animal experiments in Cancer Research’ (1999), ‘Immunization of Laboratory Animals’ (2000) and ‘Safeguarding the welfare of Lab Animals’ (2001). Anyone working with lab animals must comply with these codes.
In addition, the Dierexperimentencommissie (DEC) of the UG has formulated internal guidelines to standardize University practices. These guidelines comprise the University’s opinions about the discomfort codes, the choice of species and ethical considerations.
Animal experiments: from application to execution
CCD
The Centrale Commissie Dierproeven (CCD) is a national committee which makes decisions to grant or reject project licenses for experiments based on the research applications. On its website, the CCD publishes non-technical summaries of the licenses it has granted.
NCad is another important national committee. NCad’s role is to bring about improvements in the application of the 3R principle and the ethical assessment thereof in scientific and applied research and in teaching activities, in order to minimize the use of laboratory animals both nationally and internationally.
DEC
The UG has an impartial animal experiments committee (DEC–RUG) which assesses the use of lab animals under the auspices of the CCD, using the CCD’s opinions and guidelines. It also abides by generally applicable viewpoints from the various codes of practice. The DEC–RUGmembership includes experts in laboratory animals and their protection, animal experiments, alternatives for such experiments and ethical assessment.
The DEC assesses all research proposals in the light of current legislation and regulations. It also weighs the benefits of animal experiments against the discomfort caused to the animals to be used.
The intrinsic value of each animal is central to the decision whether an animal experiment is ethically acceptable or not. However, other considerations also play a role, for example an animal’s psychological complexity (cf. primates), the societal status of a species based on factors such as social closeness (cats and dogs), historical value (agricultural animals) and social relationship (seals).
The UG and the UMCG do not have facilities for experiments conducted with primates, and the UG has formulated a separate point of view on this issue (in Dutch only).
IvD
An important change in the revised Wod is that institutions must combine their expertise concerning animal welfare in an Instantie voor Dierenwelzijn (IvD). The IvD assesses the animal welfare aspects of a research project that has previously been approved by the DEC and the CCD and ensures that it can be properly implemented. It also advises researchers about the application of the 3R principle and supervises the research preparations and the skills and training of the researchers involved.
The IvD membership includes a designated veterinarian, the animal facility’s Location Supervisor, a scientist and, if necessary, an external expert such as a radiation specialist or biological safety officer.
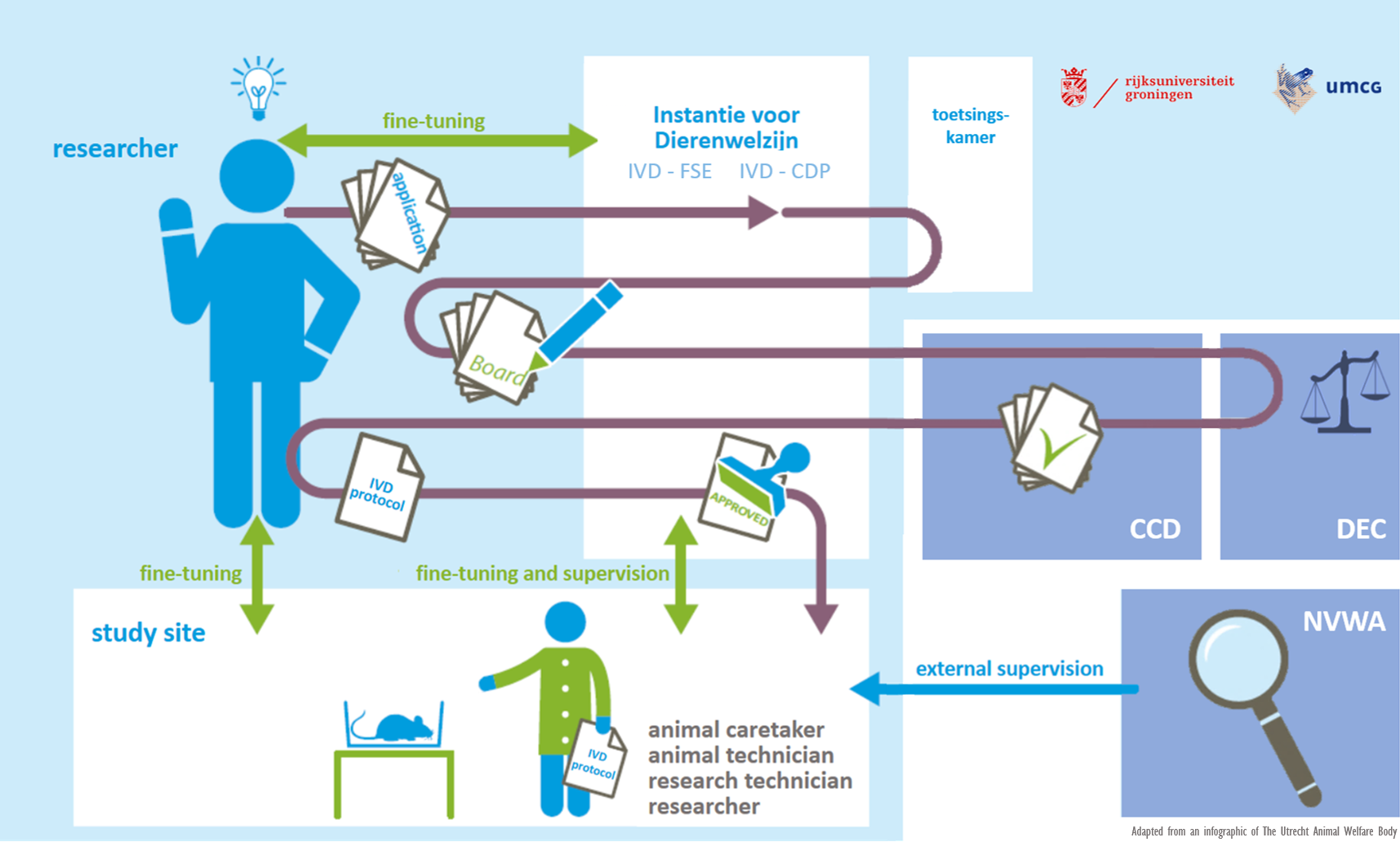
Article 14c of the Experiments on Animals Act lists the tasks of an Animal Welfare Body in five points (14c.1a to 1e). Article 14c.1c states that the IvD ‘guarantees the establishment and review of internal procedures concerning monitoring, reporting and follow-up with regard to the wellbeing of the animals housed in the institution’s animal housing facilities’. In other words, the Article states that the IvD organizes the laboratory animals’ guaranteed wellbeing and produces a record of it.
For each IvD protocol, the UG’s IvDs check whether the animal study will be carried out using animals that are caught in the wild. If so, the IvD checks whether the required flora and fauna dispensation has been obtained. This internal process will not change and continue to be used.
IvD platform
Both IvDs (Animal Welfare Bodies) of the UG are affiliated with the national IvD platform. Members of the two UG IvDs are well represented within the national IvD platform. They are active in their role as the IvD platform’s secretary and in various working groups: Education & Training, Antibiotics Policy, and Breeding Coordinators. The IvD platform is part of DALAS (Dutch Association for Laboratory Animal Science) and was very active in 2022. The IvD platform still represents more than 90% of all IvDs in the Netherlands. In addition to monthly consultations, the IvD platform has been able to maintain contacts with various government organizations such as the CCD (Central Authority for Scientific Procedures on Animals), NCad (National Advisory Committee for Animal Research Policy), LNV (Ministry of Agriculture, Nature, and Food Quality), and NVWA (Netherlands Food and Consumer Product Safety Authority). The aim of the past year was to meet with the various government organizations at least once a year to discuss any issues that were relevant to the workplace. In addition to being a discussion partner with the various government organizations, the IvD platform also wants to ensure the sharing of knowledge with the IvDs.
Within the IvD platform, 4 working groups are active: Antibiotics, Experimental Design & Statistical Analysis, Breeding coordinators and Intercollegiate Auditing. Output from these working groups will be shared with the workplace via the IvDs.
This year, the IvD platform organized the “Harry Blom consultation” in collaboration with the NCad as a one-off event. The aim of this consultation is to highlight dilemmas in current research practice from different points of view. The topic this time was “Natural behavior of the laboratory animal affects animal experiments”. Several experts shared their research findings and vision on this. The IvD Platform itself has organized an afternoon for IvDs with the theme: ‘Hearing, seeing and talking to each other’. How can we research and improve together!
Another important topic within the IvDs is the Codes of Practice (CoPs). That is why the IvD platform has taken the initiative to develop new CoPs and update old CoPs, together with the NCad. Two people from IvD-Groningen participated in the working Group on Pre- and Postoperative Care to draw up this CoP for good surgical practice.
End of experiments
Adoption
Under the revised Wet op Dierproeven, the IvD advices on adoption arrangements, including advice on appropriate socialization of the animals to be released for adoption. The license holder is of the opinion that it is not in the interest of the laboratory animals and private individuals to adopt laboratory animals. Animals kept at the UG under semi-natural conditions, including birds and fish, are an exception to this. These animals can be adopted by private individuals. The license holder does not allow such animals to be traded. In 2022, 4 Bankiva fowl, 21 Zebra finches, 43 Rainbowfish, 2 Peacock Bass, 23 Kribensis cichlids and 4 Angelfish were adopted, 263 Sticklebacks were released into nature.
Euthanasia
In most cases adoption is not possible, for example because the brain and/or other organs and body parts are required for further study and analysis. In that case, the animals will be euthanized at the end of the experiment. This is a step which neither animal carers nor researchers take lightly. The most common euthanasia procedure is one which the animals hardly notice. They are placed in a box containing a mixture of oxygen (O2) and carbon dioxide (CO2). Then the CO2 concentration is slowly increased, causing the animals to gradually lose consciousness and then pass away peacefully. Sometimes the nature of the experiment requires a different euthanasia procedure. In such cases, too, the method chosen must result in the least possible discomfort for the animal. In some cases, animals develop complications over the course of an experiment, which may lead them to suffer more than expected. Researchers will then apply the principle of the humane end point. They will remove the animal from the experiment when its suffering threatens to become unacceptable and then euthanize it to prevent further suffering.
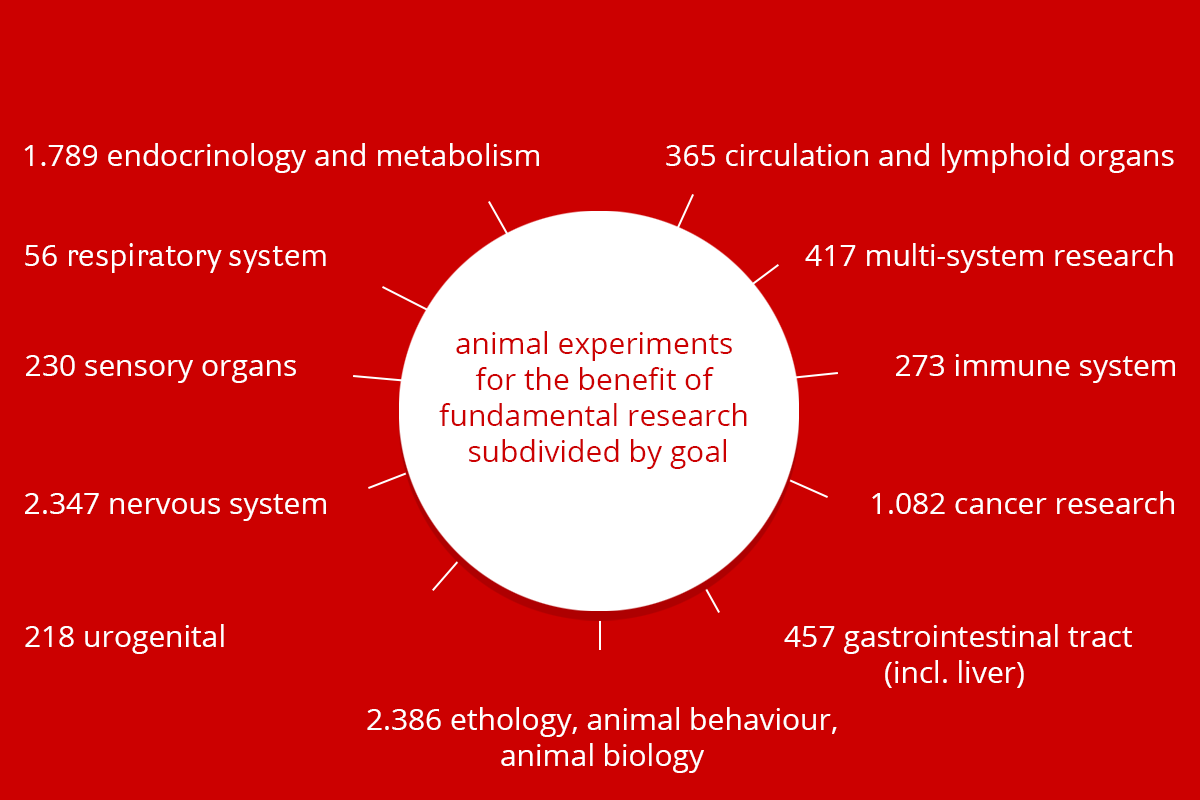
Aims of animal experiments
By far the most animal experiments were carried out to help answer a scientific question. The figure below indicates what these questions entailed. In addition to answering scientific questions, animal experiments were also conducted within the scope of teaching and training, involving, for example, students and animal technicians.
Table Zodoende
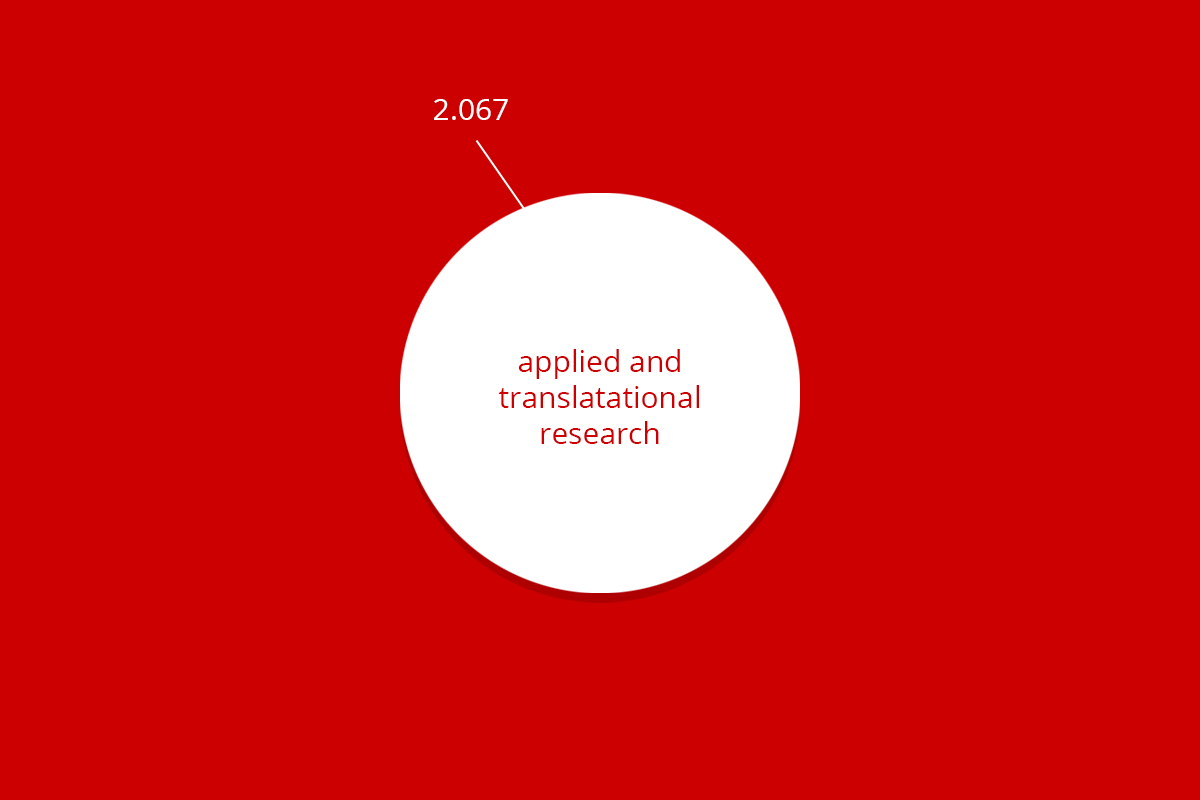
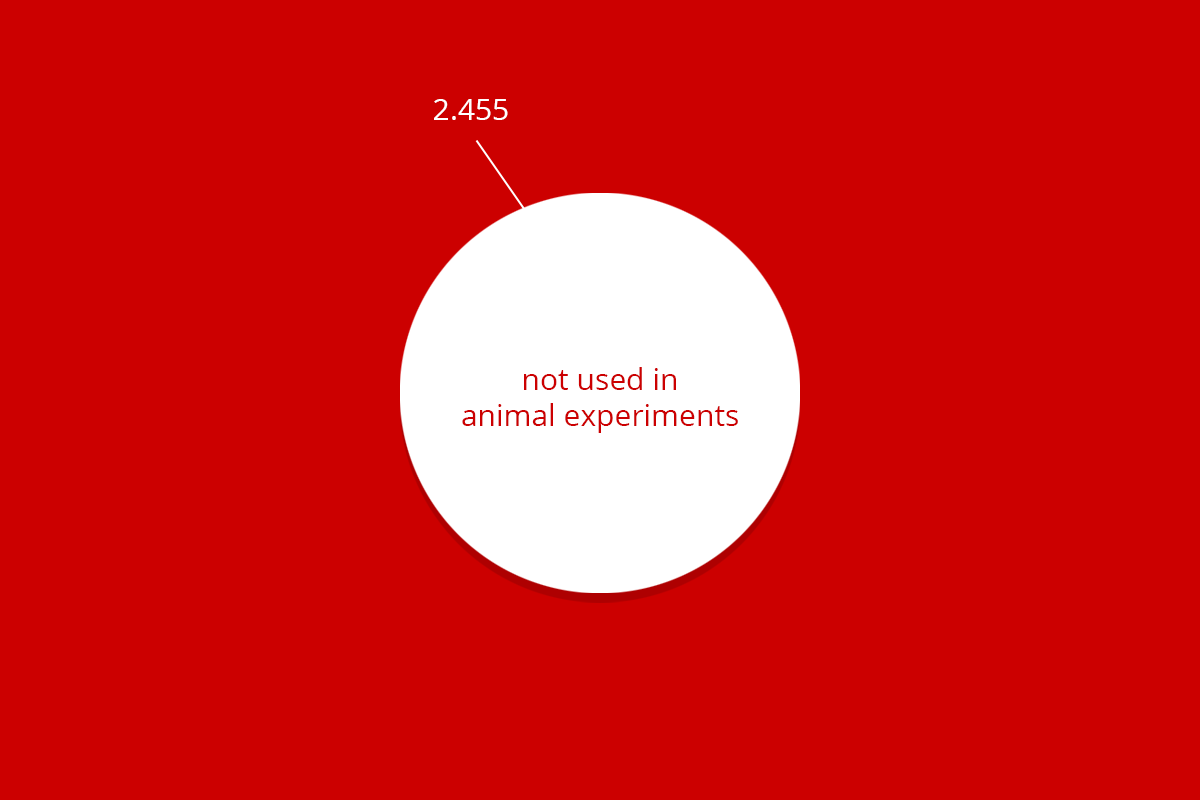
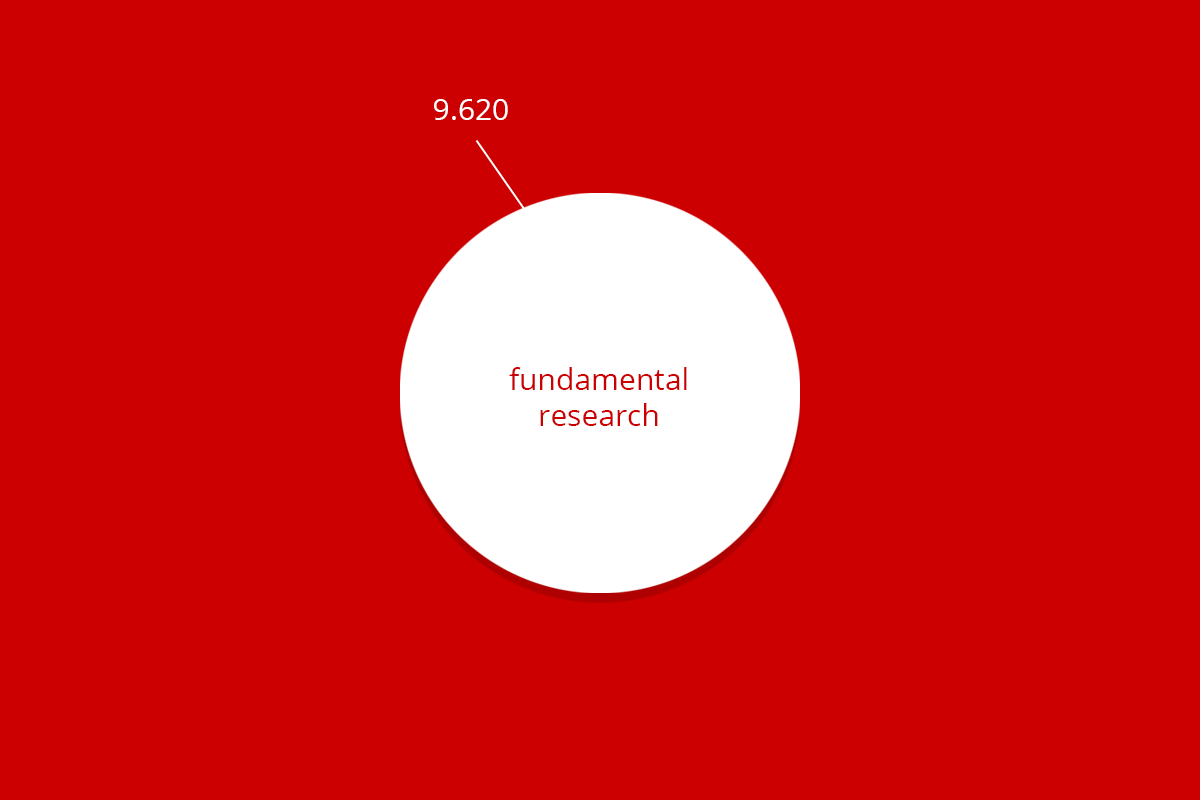
| RuG 10500 | Fundamental research | Applied and translational research | Protection of animal species | Education | Breeding with discomfort not used | Total |
| Mice | 6.055 | 1.796 | 204 | 2.455 | 10.508 | |
| Rats | 1.088 | 61 | 232 | 1.381 | ||
| Guinea pigs | 210 | 2 | 212 | |||
| Other rodents | 126 | 126 | ||||
| Other mammals | 5 | 5 | ||||
| Other birds | 1.419 | 24 | 1.443 | |||
| Xenopus | 111 | 111 | ||||
| Zebrafish | 0 | |||||
| Other fish | 816 | 816 | ||||
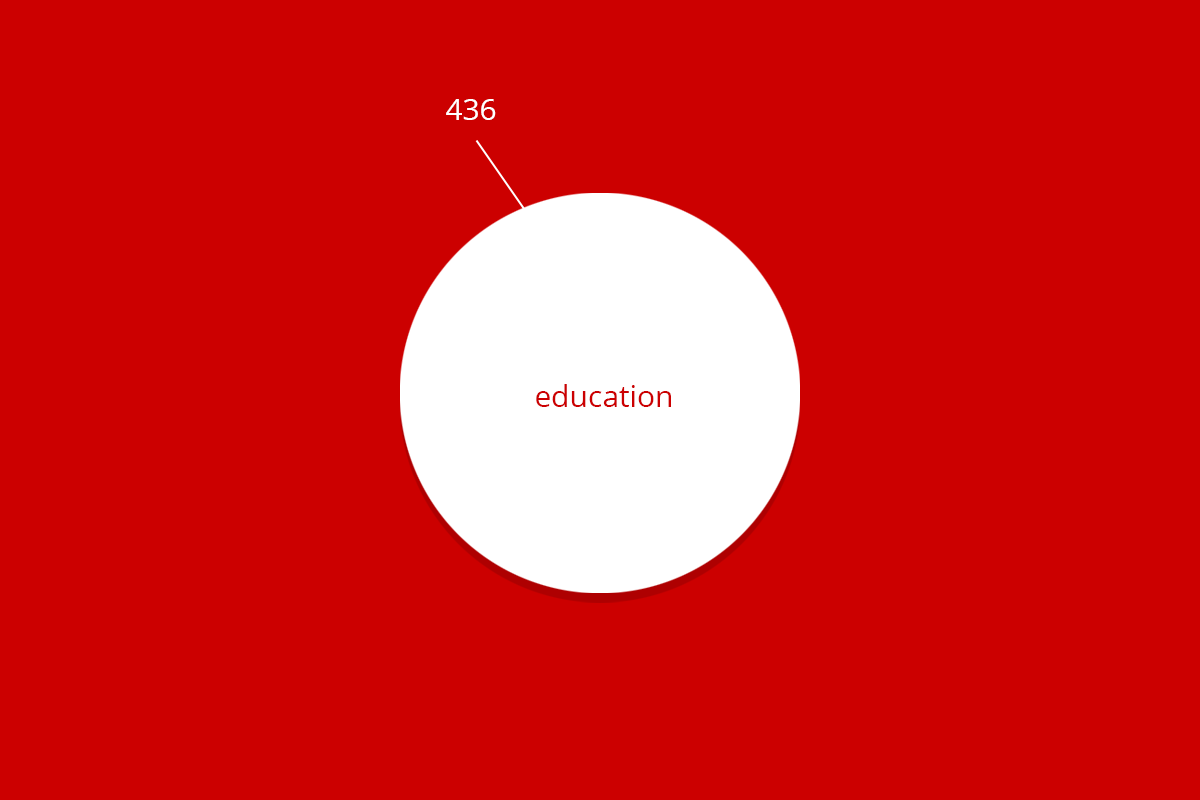
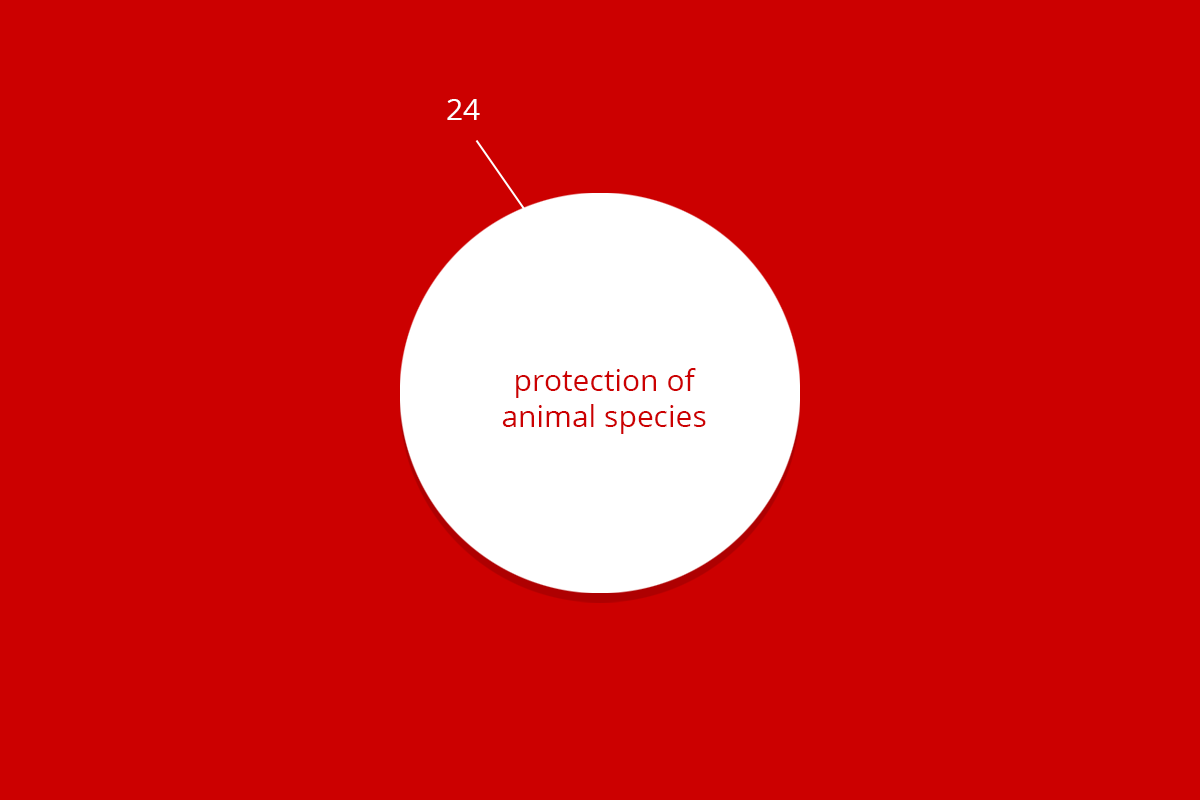
| Total | 9.620 | 2.062 | 24 | 436 | 2.455 | 14.602 |
NB empty rows and columns are not shown.
The tabel does not include animals that have been terminated without prior procedures.
Discomfort
Lab animals will always experience some degree of discomfort. The revised Wod divides discomfort into four categories. Discomfort need not take the form of pain; stress and anxiety are also regarded as discomfort. The table below shows the degrees of discomfort and the percentages of animals involved in 2022.
%
Termination
%
Mild discomfort
%
Moderate discomfort
%
Substantial discomfort
%
More than substantial discomfort
Breeding efficiency
The UG and the UMCG breed animals themselves, particularly (transgenic) mice and rats. Not all bred animals end up in an experiment. We call these animals ‘surplus animals’ or ‘breeding surplus’.
A breeding surplus is unfortunately unavoidable. Animals in an experiment often have to be as identical as possible in order to obtain reliable research results. For example, they must be the same age and sex or born under identical circumstances. Also, not all animals possess the desired genetic characteristics. For an experiment involving 60 identical transgenic mice, for example, as many as 170 mice may have to be bred: read more about this on the website of the Stichting Informatie Dierproeven. In addition, a substantial part of the breeding is needed to maintain unique or valuable breeding lines.
Reducing the number of animals that have been bred but not used in an experiment is a high priority for the University of Groningen and the Dutch government. In 2022, as in 2020 and 2021, about a fifth of the animals bred were used in experiments. For non-transgenic lines, this percentage is higher, because all offspring can be used for experiments. In transgenic lines, some of the offspring have a genotype that is not useful in experiments. The total number of animals bred (mice and rats) in 2022 is more than 4,000 lower than in 2021 and the absolute number of bred animals that were terminated without being used in an experiment is about 5000 animals lower than in 2021 and already 15000 lower than in 2020. This is going in the right direction, but the University of Groningen is well aware that the number of unused animals is still too high and more efforts are continued to be made to reduce the number of animals killed in stock.
The University of Groningen and the UMCG are at the forefront of increasing breeding efficiency through clear communication between researchers and animal caretakers (supply and demand) and reducing the breeding surplus by focusing on cryopreservation of breeding lines that are no longer actively used.

Cryopreservation
Cryopreservation is a technique in which eggs or sperm from a breeding line that is not needed for a longer period of time are frozen instead of keeping the line alive. When the breeding line is needed again, a fertilized egg is inserted into a pseudopregnant female. In the meantime, no animals are needed to maintain the line.
Since 2020, the UG has been offering to cryopreserve inactive breeding lines (lines that are no longer used for animal experiments). Twenty-eight mouse lines stopped in 2022. Six new lines started in 2022. These numbers are much lower than last year, probably because in the past two years, when the cryopreservation option became available, more lines have already been stopped.
For fish breeding lines, it is not yet possible to freeze genetic material and later use it again to breed fish. This is being tested at various institutes and the UG will keep a close eye on this trend to also enable cryopreservation of fish breeding lines in the future. All in all, we are not yet satisfied with these figures and hope to increase the percentage of unused animals in the coming years.
Replacement, Reduction, Refinement
The UG and the UMCG apply the 3R principle to research and teaching involving laboratory animals: replacement and reduction of the number of animals and refinement of the experiments in which they are used. Essentially, this means that we use as few animals as possible and conduct animal-free experiments whenever possible. Furthermore, we try to minimize the discomfort experienced by the animals. The Animal Welfare Committee (IvD) helps researchers to put these guidelines into practice.
Replacement
Researchers are only allowed to conduct an animal experiment if there are no other options. Where possible, we use alternatives to animal experiments in teaching and research, replacing laboratory animals with invertebrates, cells, tissues, computer simulations, video training or slaughterhouse material.
Reduction
Efforts must be made to reduce the number of animals required in each experiment through a research design specifying the minimum number of animals necessary to achieve reliable findings. This can be achieved, for example, by using standard strains so that the results are more comparable or by conducting a pilot study first.
Sometimes lab animals can be used again after the original experiment, in a follow-up or unrelated experiment or in a teaching activity. In 2022, 3.5% of animals was used again.
Refinement
Researchers, animal carers, animal technicians and designated veterinarians are always trying to refine all aspects of animal use and animal welfare. Optimum accommodation and adequate application of research techniques and anaesthesiology should minimize the animals discomfort. Social animals such as rats, for example, are kept in groups, which reduces their stress levels.
By refining animal experiments, we improve the animals’ welfare, which is not only good for them but benefits the quality of research too.
Applying the 3R principle in teaching activities involving lab animals
Training
The Centrale Dienst Proefdieren (CDP) applies the 3R principle as much as possible when lab animals are used for teaching purposes. When inexperienced students are first introduced to a technique, synthetic materials are used as much as possible. Students learning to suture, for example, first practice on a piece of chamois leather.
Microsurgical techniques are first practised using a piece of latex glove under the microscope and then on artificial vessels. If the students’ hand-eye coordination is sufficiently developed, they are allowed to continue with live rats. Synthetic materials are thus used whenever possible. Ultimately, however, the technique to be mastered must be practised in a live organism, since a living animal presents students with a system that is too complex to mimic with synthetic materials. To further reduce the number of animals used, instruction videos have been produced for all relevant biotechnical procedures covered during student training, so that no animals have to be used to demonstrate the techniques. Animals used in teaching are always anaesthetized before an invasive surgical procedure and are euthanized before the anaesthesia wears off, to prevent unnecessary discomfort. By thoroughly training the staff involved in animal experiments, the CDP aims to improve the quality of animal experiments and the animals’ welfare.
Anatomy practical
All Bachelor students of Biology take an anatomy and physiology practical involving the dissection of a rat. Until 2015, these rats were always euthanized shortly before the start of the practical and presented untreated to the students because this procedure results in the best specimens. In frozen and subsequently thawed specimens, certain essential structures proved difficult to see. Over the year, there are sufficient surplus animals available from breeding and invasive and non-invasive experiments to meet the needs of this practical. From the perspective of animal welfare, however, it is undesirable to keep these animals alive until the start of the practical. This is also uneconomical. For this reason, until 2015, the rats used were purchased from a commercial breeder. Despite the fact that almost all these animals were surplus animals from their breeding lines, this situation was less than ideal, not least because of the stress caused to the animals, for example during transport.
For these reasons, 2016 witnessed the start of a highly successful pilot in which surplus rats from our own breeding programme and experiments were embalmed. These embalmed rats proved highly useful in the practical because all the important structures were preserved well, which was not the case with the frozen specimens.
To embalm these rats, the Fix for Life method developed by Leiden University Medical Centre was used. This method employs an embalming fluid which is (virtually) free of the toxic and irritant substances such as formaldehyde and phenol that are commonly used to preserve tissues. This makes the method extremely suitable for teaching purposes. Another advantage is that the embalming fluid has a less offensive smell.
Embalming the rats has proven a win-win situation. First, rats no longer have to be purchased and transported, and our own surplus animals can now serve a useful purpose. At the same time, the specimens have proven extremely suitable for the practical, and their use is less taxing on students.
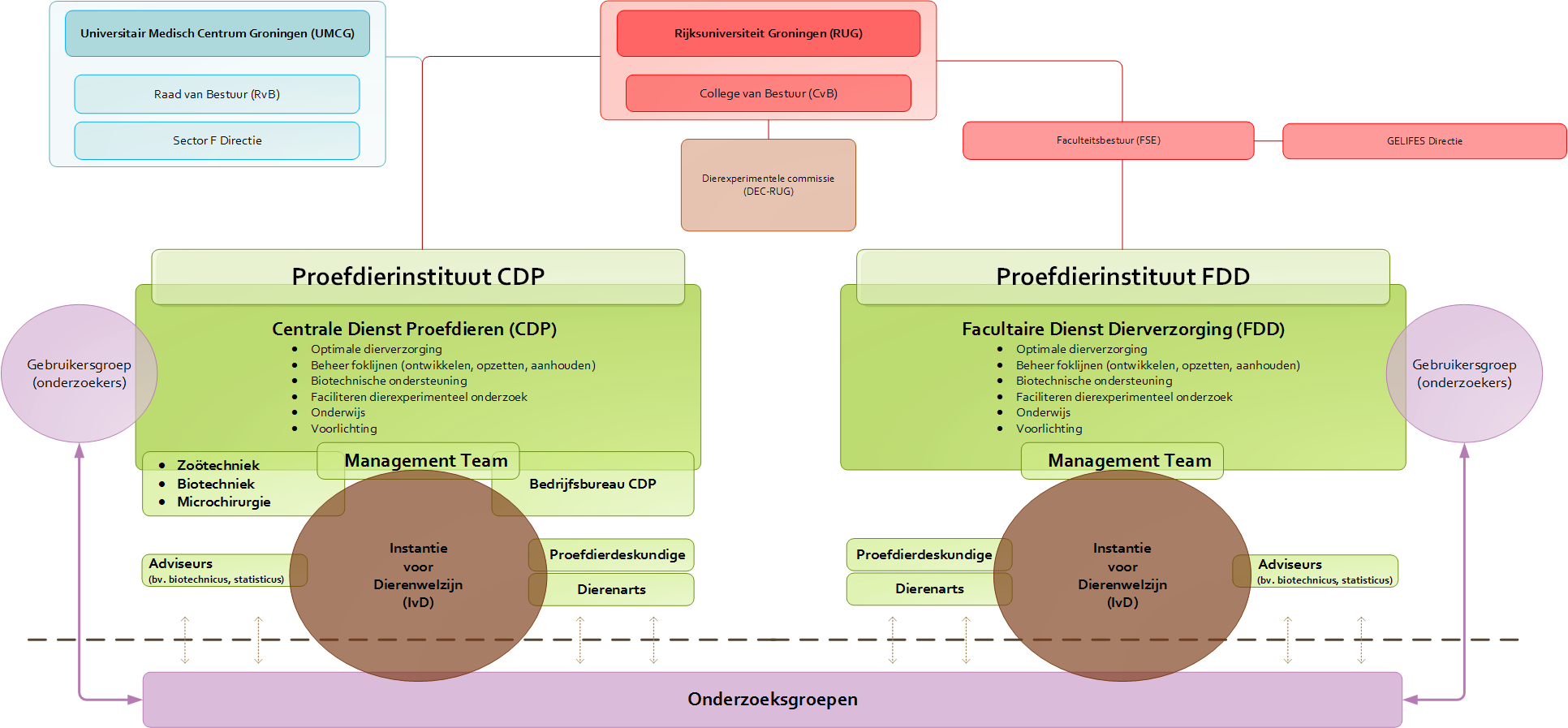
Organization and facilities
To guarantee optimum animal care and effective research, two modern animal experiment facilities have been set up: CDP at the UMCG and the Facultaire Dienst Dierverzorging (FDD) at the Linnaeusborg.
All animal studies at the UG and the UMCG are conducted either in nature or in one of the laboratories with special animal testing facilities. We take the utmost care to provide the best possible accommodations for lab animals, since they will live out almost their entire lives there. Providing accommodation thus involves more than simply meeting the statutory requirements. The CDP and the FDD have been completely renovated in 2009 and 2011, respectively, and are now among the most modern facilities in Europe. The temperature, lighting and atmospheric humidity in the animal quarters can be precisely controlled.
Inspections by the NVWA
In 2022, the NVWA did not carry out any inspections at the animal facilities.

About the University of Groningen
The University of Groningen is a research university with a global outlook, deeply rooted in Groningen, City of Talent. The UG is in the top 100 of several important ranking lists. It is very popular with its 30,000 students and staff (5250 FTE) from the Netherlands and abroad, who are encouraged to make the most of their abilities. Talent is nurtured, and the keyword is quality. The University is committed to actively cooperating with its partners in society, with a special focus on its research themes Healthy Ageing, Energy and Sustainable Society.